Research
Body mass index and cognitive decline in mild cognitive impairment individuals in north Indian population

Body mass index and cognitive decline in mild cognitive impairment individuals in north Indian population
Dr. Rohit Rao Pushkar1, Dr. Vineeta Singh2*
Abstract:
Background: Midlife individuals with a higher body mass index (BMI) are at an increased risk of experiencing cognitive decline, changes in brain structure, and developing Alzheimer’s disease (AD). Mild cognitive impairment (MCI) represents an early stage of cognitive decline that may progress to AD. This study aims to explore the association between BMI and cognitive dysfunction in individuals with MCI.
Methods: A total of 200 elderly individuals with memory complaints were screened for MCI at the Sir Sundar Lal Hospital, Varanasi, between December 2015 and September 2017. Among them, 37 individuals with MCI and 37 age- and sex-matched healthy controls were selected for detailed investigation. Cognitive function was assessed using the HMSE and MOCA scales, along with a series of neuropsychological tests. Data were analyzed using SPSS version 16, with significance set at p < 0.05.
Results: In this study neuropsychological assessments revealed significant impairments in attention, verbal fluency, memory, and constructional ability in the MCI group compared to controls. Correlation analysis showed a positive relationship between BMI and cognitive scores, while age was negatively correlated with cognitive function.
Conclusion: This study suggests that low BMI and cognitive dysfunction are associated with psychological alterations in MCI individuals, which could impair daily living activities.
Keyword: Body Mass Index, Mild cognitive impairment, Alzheimer’s disease, Diagnostics biomarker, Differential biomarker
Introduction
Increased risk of Alzheimer disease (AD) in later life, cognitive decline, and structural alterations in the brain are all linked to higher body mass index (BMI) in midlife [1, 2]. In clinical research, the term "mild cognitive impairment" (MCI) is used to characterize a subset of older people who have cognitive impairment but whose memory loss is not severe enough to be diagnosed as dementia. A cognitive impairment that may lead to AD is known as mild cognitive impairment, or MCI [3]. Previous literature reported that, the annual rate of MCI to AD conversion is 10– 12% however only 1– 2% of the elderly population is with AD [4]. A Kolkata based population study by Das et al., 2007 have shown 12 % prevalence of MCI in elderly population [5]. Prior diagnosis and intervention of MCI could prevent or delay the onset of AD. In the present scenario, it is difficult to diagnose potential changes, which is involved in the development of MCI and progression to AD. It was earlier reported that cognitive decline is related to metabolic status of the human body.
A prior research found that those who were overweight or obese had a lower risk of developing dementia and AD [6]. Nonetheless, some research has demonstrated that obesity triggers the beginning of cognitive impairment [7, 8]. The MCI group in this study is made up of people with a variety of demographic traits and lifestyles. We hypothesized that an individual's age, sex, status with cognitive interventions, or presence of chronic conditions could all have different effects on their BMI. As a result, for those with MCI, the effect of BMI on the start of AD may differ. In the current study, we looked for a relationship between BMI and different types of cognitive dysfunction in people with motor cortex injury.
Materials and Methods
Case and control
The study encompassed 2000 senior citizens who had reported instances of forgetfulness in the outpatient and inpatient departments of Sir Sundar Lal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi. The assessments were performed from December 2015 to September 2017 by qualified medical professionals and mental health specialists. Two hundred senior people were screened for MCI, and 200 of them were confirmed to have the condition. Since only 37 MCI patients were enrolled in our follow-up study, we looked at them in great depth. Age and sex matched control group of thirty-seven healthy persons.
Psychological screening
The modified Petersen's criteria were used to identify MCI [8]. Higher HMSE/MOCA scores were indicative of superior cognitive abilities; the values ranged from 0 to 30 [9, 10]. 37 people who claimed to be forgetful were evaluated psychologically.
Statistical Analysis
With the use of SPSS version 16, statistical tools were used to analyze all of the collected data. Continuous data that were normally distributed were examined using the independent-samples t-test and displayed as mean ± SD. The frequency of the categorical data was expressed, and the chi-square test was used for analysis. A p-value of less than 0.05 was deemed statistically significant. Prism 8.0 was used to analyze the area under the curve.
Results
In the present study, we found that the mean HMSE was 24.65 in case group and 29.00 in control group. HMSE was low in case group. MOCA was 22.16 in case group and 29.00 in control group. MOCA was low in case group as compared to control group. We also found that in MCI individuals BMI was found 25.18 and 23.68 in control individuals. The weight of MCI individuals were 69.10 (kg) and 66.18 (kg) in control individuals (Table 1).
Table: 1 Demographic and other details.
|
|
Control |
MCI |
|
Age |
59.0 |
59.92 |
|
Gender |
33/4F |
33/4 F |
|
BMI |
23.68 |
25.18 |
|
Weight |
66.13 |
69.18 |
|
HMSE |
29.0 |
24.65 |
|
MOCA |
29.0 |
22.16 |
Further, neuropsychological examination showed that attention forward (Normal= 5-7) was impaired in 1 out of 37 MCI individuals, overall MCI cases had lower digit span as compared to control group. Attention backward more impaired in case group as compared to control group. Verbal similarities is more impaired in case group (23 out of 37) as compared to control group (5 out of 37). Verbal fluency is more impaired in case group (37 out of 37) as compared to control group (2 out of 37). Proverb interpretation is more impaired in case group (1 out of 37) as compared to control group (0 out of 37). Environmental autonomy, judgment, Insight was intact in both the groups. Constructional ability 3D was more impaired in case group (7 out of 37) as compared to control group (0 out of 37). Clock drawing was more impaired in case group (2 out of 37) as compared to control group (0 out of 37). Daisy in flowerpot was more impaired in case group as compared to control group. Calculation verbal simple was intact in both the groups. Calculation verbal complex was more impaired in case group (8 out of 37) as compared to control group. Recent memory orientation was more impaired in case group (6 out of 37) as compared to control group. Remote memory was intact in both the groups. New learning ability was more impaired in case group as compared with control group. Visual memory was more impaired in case group as compared with control. In above, all the alteration was found significant.


However, motor luria is more impaired in case group (4 out of 37) as compared to control group (1 out of 37). Graphic luria is more impaired in case group (2 out of 37) as compared to control group (0 out of 37). Conflicting Instruction is more impaired in case group (3 out of 37) as compared to control group (0 out of 37). Go No Go test is more impaired in case group (1 out of 37) as compared to control group (0 out of 37). Calculation written complex was more impaired in case group (3 out of 37) as compared to control group. Lobar function occipital was intact in both the groups. In above, all the alteration were not found significant (Table 2).
Table: 2 Neuropsychological examination.
|
Variables |
Variable Parameter |
Group1 (Case) |
Group 2 (Control) |
P-value |
|
Sex |
F M |
4(50%) 33(50%) |
4(50%) 33(50%) |
p-1.0 |
|
Attention FW |
4(Digit span) 5(Digit span) 6(Digit span) 7(Digit span) |
1(100%) 16 (80%) 20(52.6%) 0(0%) |
0(0%) 4(20%) 18(47.4%) 15(100%) |
p<0.001 |
|
Attention BW |
3(Digit span) 4(Digit span) 5(Digit span) 6(Digit span) |
14(100%) 17(100%) 6(23.1%) 0(0%) |
0(0%) 0(0%) 20(76.9%) 17(100% |
p<0.001 |
|
Trail A |
1 (< 78 sec) 2 (>78 sec) |
19(35.8%) 18(85.7%) |
34(64.2%) 3(14.3%) |
p<0.001 |
|
Trail B |
1 (<273 sec)
2 (>273 sec) |
30(44.8%) 44.8% 7(100%) |
37(55.2%) 55.2% 0(0%) |
p<0.01 |
|
Motor Luria |
1 (Fail alone but perform with examiner) 2 (3 correct series) 3 (6 correct series) |
1(100%)
3(75%) 33(47.8) |
0(0%)
1(25%) 36(52.2%) |
p=0.345 |
|
Graphic Luria |
Impaired Normal |
2 (100%) 35(48.6) |
0(0%) 37(51.4%) |
p=0.152
|
|
Verbal Similarity |
0 8 10 |
1(100%) 22(81.5) 14(30.4%) |
0(0%) 5(18.5%) 32(69.6%) |
p=0.000
|
|
Verbal Fluency |
1 (3-5 word) 2 (6-9 word) 3 (>9 word) |
12(100%) 25(92.6%) 0(0%) |
0(0%) 2(7.4%) 35(35%) |
p=0.000 |
|
Conflicting Instruction |
2 (1-2 error) 3 ( No error ) |
3(100%) 34(47.9%) |
0(0%) 37(52.1%) |
p=0.077
|
|
Go-No-Go test |
1 (>2 error) 3 ( No error ) |
Frequency Frequency |
1(100%) 36(49.3%) |
p=0.314
|
|
Proverb Interpretation |
4 (< 5 is abnormal) 8 9 10 |
1(100%) 26(66.7%) 8(100%) 2(7.7%) |
0(0%) 13(33.3%) 0(0%) 24(92.3%) |
p=0.001 |
|
Environmental, Autonomy, Judgement, Insight
|
Intact |
37(50.0%) |
37(50.0%) |
p=1.0 |
|
Neglect (Visual, Tactile, Auditory) Line bisection Word Cancellation Simultagnosia Praxis Right Left Orientation Finger Agnosia Geographic Orientation Cortical Sensation |
Normal |
37 (50.0%) |
37 (50.0%) |
p=1.0 |
|
Constructional Ability 2D |
Intact |
37(50.0%) |
37(50.0%) |
p=1.0 |
|
Constructional Ability 3D |
Impaired Normal |
7(100.0%) 30(44.8%) |
0(0%) 37(55.2%) |
p=0.005
|
|
Clock Drawing |
4 5 6 |
2 (100%) 10(100%) 25 (40.3%) |
0(0%) 0(0%) 37 (59.7%) |
p=0.001
|
|
Daisy in flower pot |
Excellent Fair |
0 (0%) 30 (100%) |
3(100%) 0(0%) |
p=0.00 |
|
Calculation Verbal Simple |
Normal |
37(50%) |
37(50%) |
p=1.0 |
|
Calculation Verbal Complex |
Impaired Normal |
8(100%) 29 (43.9%) |
0(0%) 37(56.1%) |
p=0.003 |
|
Calculation Written Complex |
Impaired Normal |
3(100%) 34(47.9%) |
0(0%) 37(52.1%) |
p=0.077 |
|
Recent Memory (Orientation) Day, Date, Month, Year, Season |
Impaired Intact |
6 (100%) 34 (47.9%) |
0(0%) 37(52.1%) |
p=0.011 |
|
Recent memory (Place, Person) |
Impaired Intact |
9 (100%) 28(43.07%) |
0(0%) 37(56.92%) |
p=1.0
|
|
Remote memory |
Impaired Intact |
12(100%) 15(31.3%) |
0(0%) 37(68.7%) |
12(100%) 52(100%) |
|
New Learning ability |
Impaired Intact |
27 (100%) 10(21.3%) |
0(0%) 37(78.7%) |
p=0.001 |
|
Visual Memory |
Impaired Intact |
7(100%) 30(44.8%) |
0(0%) 37(55.2%) |
p=0.005 |
|
Lobar Function Occipital |
Intact |
37(50%) |
37(50%) |
p=1.0 |
Correlation study showed that height (0.075, 0.112), weight (0.168, 0.105) and BMI (0.085, 0.023) is positively correlated with the HMSE and MOCA score respectively. However, age (-0.145, -0.211) is negatively correlated with the HMSE and MOCA score (Table 3).
Table: 3 Pearson Correlation Coefficient in of psychological screening with other factors.
|
S. No. |
Factors |
HMSE |
MOCA |
|
1. |
Height |
0.75 |
0.112 |
|
2. |
Weight |
0.168 |
0.106 |
|
3. |
BMI |
0.085 |
0.023 |
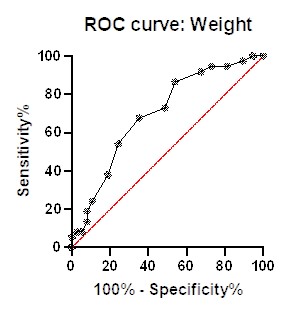
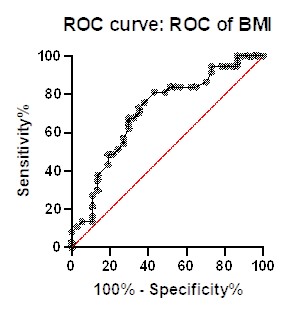
Diagnostic potential analysis showed that weight with area under curve 0.6994; 95% confidence interval at 0.5795 to 0.8193 with p value 0.032 and BMI with area under 0.7115; 95% confidence interval at 0.5926 to 0.8303 with p value 0.017.
Discussion
The purpose of this study was to determine whether BMI was related to any particular cognitive dysfunction in people with motor cortex injury. This study demonstrated that the MCI group's HMSE score was lower than the control group's. A similar finding—that MCI patients had lower MMSE scores than healthy control people—was previously published by Onwuekwe in 2012 [11]. The degree of impairment is correlated with the HMSE and MMSE scores, respectively, according to Ganguli et al., 2004; Tripathi et al., 2015; and Folstein et al., 1975 [12–14]. In comparison to the control group, the MCI group's MOCA score was lower. According to Freitas et al., 2004 and McLennan et al., 2011 MoCA comes as better cognitive screening test for milder forms of cognitive impairment, having surpassed the well-known limitations of the MMSE [15, 16].
In the present study, we further performed the detailed neuropsychological examination and categories the based on the brain lobes. Present study showed, attention forward (Normal= 5-7) was impaired in 1 out of 37 MCI and overall MCI cases had lower forward digit span as compared to control group and minimum score obtained was 4, while the maximum score was 6. Attention backward was more impaired in case group as compared to control group with minimum score obtained was 3, while the maximum score was 5. Attention is the individual’s ability to attend a specific stimulus without being distracted by extraneous internal and environmental stimuli [16]. Previous study also stated that MCI individuals have complaint of impaired attention [8].
In comparison with the control group (14.3%), the case group's Trail A test performance is worse. In comparison to the control group, the case group's Trail B test performance is worse. This exam is a well-liked way to assess visual seeking, perceptuomotor speed, attentiveness, and the capacity to switch concepts quickly. It is a part of the Halstead-Reitan Battery [17, 18]. When interpreting the trail-B test, both speed and accuracy are taken into account. Individuals with frontal lobe lesions appear to perform considerably worse than those with lesions in other loci, according to the Trail B test, which is sensitive to the effects of brain dysfunction of any kind [19]. A prior study also revealed that people with MCI had trouble constructing trails [20, 21].
Studies of correlation have shown that age is adversely correlated with HMSE and MOCA scores, although height, weight, and BMI are positively correlated. A positive relationship between BMI and cognitive performance was found in 2016 by Kim et al. in people with mild cognitive impairment (MCI). According to their research, MCI patients had greater BMIs and body weights than healthy controls. In a similar vein, a 2018 study by Cronk et al. discovered that people with lower baseline BMI had a higher rate of cognitive impairment in MCI. This finding raises the possibility that body composition influences MCI and/or influences the rate of cognitive deterioration. According to Joo et al. (2018), MCI patients who are underweight have a higher chance of getting dementia.
Conclusion
Individuals with cognitive impairment was present with low BMI level and are able to perform their daily living activity. These individuals were evidenced with low HMSE/MOCA score, which is positively correlated with the BMI. These elderly MCI individuals’, pertain decline in recent memory and reduced constructional ability. Present study showed that, low BMI and cognitive dysfunction is associated with the psychological alteration, which affect MCI individual task or performance. Such alterations in neuropsychological examination, with low BMI might use for the identification of MCI in the elderly population.
Ethical Approval and consent for participation
Ethical approval and consent for participation has been taken from each participant.
Competing interests
None
Data Availability
Data will be available on request.
References
1. Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005 Jun 11;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. Epub 2005 Apr 29. PMID: 15863436; PMCID: PMC558283.
2. Kim S, Kim Y, Park SM. Body Mass Index and Decline of Cognitive Function. PLoS One. 2016 Feb 11;11(2):e0148908. doi: 10.1371/journal.pone.0148908. PMID: 26867138; PMCID: PMC4751283.
3. Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015 Jul 28;85(4):331-8. doi: 10.1212/WNL.0000000000001788. Epub 2015 Jul 1. PMID: 26136516.
4. Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006 Jan 24;66(2):223-7. doi: 10.1212/01.wnl.0000194507.39504.17. PMID: 16434658; PMCID: PMC2860622.
5. Das SK, Bose P, Biswas A, Dutt A, Banerjee TK, Hazra AM, Raut DK, Chaudhuri A, Roy T. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007 Jun 5;68(23):2019-26. doi: 10.1212/01.wnl.0000264424.76759.e6. PMID: 17548552.
6. Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996 Oct;44(10):1147-52. doi: 10.1111/j.1532-5415.1996.tb01362.x. PMID: 8855991.
7. Gao S, Nguyen JT, Hendrie HC, Unverzagt FW, Hake A, Smith-Gamble V, Hall K. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc. 2011 Jan;59(1):18-25. doi: 10.1111/j.1532-5415.2010.03169.x. Epub 2010 Nov 4. PMID: 21054328; PMCID: PMC3020982.
8. Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007 Aug;25(3):577-609, v. doi: 10.1016/j.ncl.2007.03.008. PMID: 17659182; PMCID: PMC2682228.
9. Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007 Aug 21;69(8):739-46. doi: 10.1212/01.wnl.0000267661.65586.33. PMID: 17709705.
10. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695-9. doi: 10.1111/j.1532-5415.2005.53221.x. Erratum in: J Am Geriatr Soc. 2019 Sep;67(9):1991. doi: 10.1111/jgs.15925. PMID: 15817019.
11. Onwuekwe I. Assessment of mild cognitive impairment with mini mental state examination among adults in southeast Nigeria. Ann Med Health Sci Res. 2012 Jul;2(2):99-102. doi: 10.4103/2141-9248.105653. PMID: 23440002; PMCID: PMC3573524.
12. Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004 Jul 13;63(1):115-21. doi: 10.1212/01.wnl.0000132523.27540.81. PMID: 15249620.
13. Tripathi R, Kumar K, Balachandar R, Marimuthu P, Varghese M, Bharath S. Neuropsychological markers of mild cognitive impairment: A clinic based study from urban India. Ann Indian Acad Neurol. 2015 Apr-Jun;18(2):177-80. doi: 10.4103/0972-2327.150566. PMID: 26019415; PMCID: PMC4445193.
14. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189-98. doi: 10.1016/0022-3956(75)90026-6. PMID: 1202204.
15. Freitas S, Simões MR, Alves L, Vicente M, Santana I. Montreal Cognitive Assessment (MoCA): validation study for vascular dementia. J Int Neuropsychol Soc. 2012 Nov;18(6):1031-40. doi: 10.1017/S135561771200077X. Epub 2012 Jun 8. PMID: 22676901.
16. McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the montreal cognitive assessment (MoCA) as a screening test for mild cognitive impairment (MCI) in a cardiovascular population. J Geriatr Psychiatry Neurol. 2011 Mar;24(1):33-8. doi: 10.1177/0891988710390813. Epub 2010 Dec 14. PMID: 21156989.
17. Reed JC and Reed HBC. The Halstead-Reitan Neuropsychological Battery. 1997; 93–129.
18. Weiner MF, Hynan LS, Rossetti H, Falkowski J. Luria's three-step test: what is it and what does it tell us? Int Psychogeriatr. 2011 Dec;23(10):1602-6. doi: 10.1017/S1041610211000767. Epub 2011 May 4. PMID: 21554794; PMCID: PMC3399685.
19. Belleville S, Gauthier S, Lepage E, Kergoat MJ, Gilbert B. Predicting decline in mild cognitive impairment: a prospective cognitive study. Neuropsychology. 2014 Jul;28(4):643-52. doi: 10.1037/neu0000063. Epub 2014 Mar 3. PMID: 24588699.
20. Kopp B, Rösser N, Tabeling S, Stürenburg HJ, de Haan B, Karnath HO, Wessel K. Errors on the Trail Making Test Are Associated with Right Hemispheric Frontal Lobe Damage in Stroke Patients. Behav Neurol. 2015;2015:309235. doi: 10.1155/2015/309235. Epub 2015 May 13. PMID: 26074673; PMCID: PMC4444530.
21. Miskin N, Thesen T, Barr WB, Butler T, Wang X, Dugan P, Kuzniecky R, Doyle W, Devinsky O, Blackmon K. Prefrontal lobe structural integrity and trail making test, part B: converging findings from surface-based cortical thickness and voxel-based lesion symptom analyses. Brain Imaging Behav. 2016 Sep;10(3):675-85. doi: 10.1007/s11682-015-9455-8. PMID: 26399235; PMCID: PMC5786430.
22. Nishioka C, Poh C, Sun SW. Diffusion tensor imaging reveals visual pathway damage in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015;45(1):97-107. doi: 10.3233/JAD-141239. PMID: 25537012; PMCID: PMC4500052.
23. Cronk BB, Johnson DK, Burns JM; Alzheimer's Disease Neuroimaging Initiative. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010 Apr-Jun;24(2):126-30. doi: 10.1097/WAD.0b013e3181a6bf3f. PMID: 19571736; PMCID: PMC3068614.
24. Joo SH, Yun SH, Kang DW, Hahn CT, Lim HK, Lee CU. Body Mass Index in Mild Cognitive Impairment According to Age, Sex, Cognitive Intervention, and Hypertension and Risk of Progression to Alzheimer's Disease. Front Psychiatry. 2018 Apr 17;9:142. doi: 10.3389/fpsyt.2018.00142. PMID: 29719518; PMCID: PMC5913709.