Review
Sovateltide: A Promising Therapeutic Agent for Stroke Treatment – A Comprehensive Narrative Review

Sovateltide: A Promising Therapeutic Agent for Stroke Treatment – A Comprehensive Narrative Review
TT Favas , Jithin Antony Bose, Gigy Varkey Kuruttukulam, Vishal V Panicker
TT Favas , Department of Neurology ALMAS hospital Kottakkal Kerala, India
E-mail: favas87@gmail.com
Introduction
- Background
Stroke is a major global health challenge, ranked as the second leading cause of death and a principal contributor to long-term disability worldwide.1,2 With approximately 15 million people affected annually, the economic and social burden of stroke is immense, leading to significant healthcare costs and loss of productivity.2 As the global population ages and the prevalence of risk factors such as hypertension, diabetes, and obesity increases, the incidence of stroke is expected to rise, further exacerbating its impact on health systems worldwide.1,2
Ischemic stroke, the most common form, accounts for about 87% of all stroke cases and occurs when a blood clot obstructs cerebral blood flow, resulting in neuronal injury and death.3 The treatment of ischemic stroke is highly time-sensitive, with acute therapies aimed at restoring blood flow to the brain to minimize neuronal damage. The primary reperfusion therapies include intravenous thrombolysis with tissue plasminogen activator (tPA) and mechanical thrombectomy, which are effective but limited by narrow therapeutic windows and strict eligibility criteria.4,5
Despite advancements in acute stroke management, a significant proportion of patients do not receive timely intervention due to delays in hospital arrival, contraindications to tPA, or failure to meet criteria for thrombectomy.3 The typical window for administering tPA is within 4.5 hours of stroke onset, and for mechanical thrombectomy, it is within 6 to 24 hours, or more depending on imaging results.4-7 These stringent requirements leave many patients without access to potentially life-saving therapies.
Moreover, the outcome of stroke is heavily influenced by the time to treatment, with delays leading to increased neuronal death and worsened neurological outcomes.5,6 Therefore, there is a pressing need for therapeutic strategies that can be administered beyond the current time constraints and to a broader patient population, including those with contraindications for existing treatments.
The limitations of current therapies highlight the urgent need for adjunctive treatments that extend beyond the acute phase, providing neuroprotection and promoting long-term recovery. Such therapies could potentially widen the treatment window and offer benefits to a broader range of patients who currently lack viable treatment options.
- Sovateltide
Sovateltide, also known as PMZ-1620 or IRL-1620, is a novel synthetic peptide and analog of endothelin-1 and acts as a selective endothelin B (ETB) receptor agonist. It is the first drug in its class to receive approval for use, specifically targeting conditions such as acute cerebral ischemic stroke. The mechanism of action of Sovateltide involves stimulating ETB receptors, which play a crucial role in several key processes within the central nervous system. These processes include neuroprotection, where Sovateltide protects neural cells from ischemic damage by reducing apoptosis and promoting cell survival. Additionally, it enhances neurogenesis and angiogenesis, facilitating the growth of new neurons and blood vessels, which aids in the recovery of brain tissue following an ischemic event. Moreover, Sovateltide contributes to mitochondrial protection by maintaining mitochondrial function, which is essential for energy production and cell viability. This comprehensive action makes Sovateltide a promising therapeutic agent in the treatment of ischemic stroke and potentially other neurological conditions like hypoxic-ischemic encephalopathy (HIE), spinal cord injuries and Alzheimer's disease.8,9
The development of Sovateltide is based on a growing understanding of the molecular and cellular processes underlying ischemic brain injury and recovery. Unlike traditional treatments that focus solely on reperfusion, Sovateltide aims to modulate the complex pathophysiology of stroke by targeting multiple pathways involved in neuronal death, inflammation, and tissue repair. This comprehensive approach has the potential to improve outcomes for patients with ischemic stroke by providing both acute neuroprotection and long-term neurorestoration.
This narrative review explores the current understanding of Sovateltide's pharmacological profile, preclinical and clinical evidence supporting its use in ischemic stroke, and potential challenges in its clinical implementation.
- Objectives
The primary objectives of this narrative review are to elucidate the mechanisms of action of Sovateltide in the context of stroke treatment, to evaluate the preclinical and clinical evidence supporting the efficacy and safety of Sovateltide, to identify challenges and considerations in the clinical integration of Sovateltide, to propose future research directions to optimize the therapeutic potential of Sovateltide in stroke care. The review aims to provide a comprehensive synthesis of existing knowledge and propose future research directions to optimize the therapeutic potential of Sovateltide in stroke care.
Methods
The literature search for this narrative review was conducted using the PubMed, Google scholar, Scopus, Cochrane Library, ClinicalTrials.gov, and Web of Science databases. The search strategy employed keywords such as "stroke," "ischemic stroke," "acute ischemic stroke," "cerebrovascular disease," "neuroprotection," "neurorestoration," "neurogenesis," "angiogenesis," "mechanisms of action," "clinical trials," "safety," "efficacy," and "animal models" in combination with "endothelin B receptor agonist," "Sovateltide." The inclusion criteria encompassed articles published in English till July 2024. Relevant study designs included preclinical studies, clinical studies and trials, reviews, and meta-analysis focusing on Sovateltide and its application in stroke treatment. Studies that were unrelated to Sovateltide or focused on non-stroke conditions were excluded from this review. Articles were selected based on their relevance, methodological rigor, and contribution to understanding Sovateltide's therapeutic potential.
Discussion
Sovateltide is a selective agonist of endothelin B receptors (ETBRs) being developed as a novel treatment for neurovascular disorders, including ischemic stroke. This compound plays a crucial role in neuroprotection, neuroregeneration, and vascular regulation (Figure 1).9
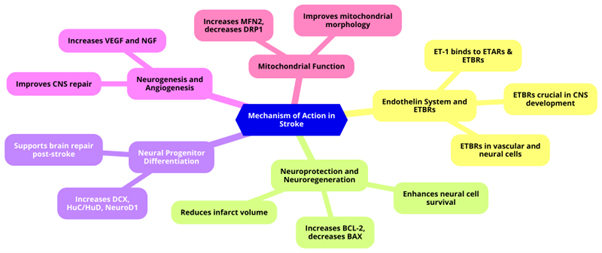
Figure 1: Mechanism of Action of Sovateltide in Stroke Treatment
- Mechanism of Action in Stroke and preclinical studies
1. Endothelin System and ETBRs
- Vascular Regulation: The endothelin system includes endothelins (ETs) and their receptors, essential for vascular regulation. Endothelin-1 (ET-1), a vasoconstrictor, can bind to endothelin A (ETARs) and endothelin B (ETBRs) receptors.10,11 Sovateltide specifically targets ETBRs.9 ETBRs are G-protein coupled receptors expressed in vascular and neural cells, playing crucial roles in CNS development, neural cell survival, and proliferation. ETBRs are critical in early CNS development, with deficiencies leading to birth defects and increased apoptosis in animal models.12,13
- Function of ETBRs: ETBRs, located on endothelial cells, are involved in vasorelaxation, cell growth regulation, and ET-1 clearance.12,13
2. Neuroprotection and Neuroregeneration
- Neural Cell Survival and Proliferation: Sovateltide enhances neural cell survival and proliferation, protecting brain tissues post-stroke. Under pathological conditions, ETBRs are expressed in neuronal progenitor cells (NPCs) and glial cells, promoting neural regeneration. In animal models, Sovateltide reduces infarct volume, oxidative stress, and enhances pro-angiogenic, pro-survival, and anti-apoptotic markers.14-18 It increased the expression of the anti-apoptotic marker BCL-2 and decreased the expression of the pro-apoptotic marker BAX, independent of nerve growth factor (NGF) levels.19
3. Neural Progenitor Differentiation
- Sovateltide enhances NPC differentiation, as indicated by increased expression of the neuronal progenitor marker Doublecortin (DCX) and differentiation regulators HuC/HuD and NeuroD1 in the ischemic brain.14
- In vitro studies with cultured NPCs exposed to hypoxia and Sovateltide showed increased differentiation and maturation, supporting Sovateltide's role in NPC differentiation. This aids in brain repair and recovery post-stroke. Studies in rats show increased expression of neural progenitor markers and decreased infarct areas following treatment.14,20
4. Neurogenesis and Angiogenesis
- CNS Repair: ETBR stimulation post-CNS damage enhances neurogenesis and angiogenesis, promoting CNS repair. As an ETBR agonist, Sovateltide improves neurological and motor functions, reduces infarct volume, and decreases oxidative stress after stroke. It also, increases expression of vascular endothelial growth factor (VEGF) and nerve growth factor (NGF).21-24
5. Mitochondrial Function
- Improved Morphology and Function: Mitochondria are crucial for neuronal survival and function. Sovateltide treatment improves mitochondrial morphology, fusion, and biogenesis, shown by increased expression of the fusion marker MFN2 and decreased expression of the fission marker DRP1 in brain tissues post-stroke.14,21
Role in Cerebral Ischemia
- Cerebral Blood Flow and Mitochondrial Health: ETBR activation by Sovateltide improves cerebral blood flow and reduces neural cell apoptosis after ischemic stroke. It promotes mitochondrial health by improving fusion, size, and biogenesis, crucial for cell survival and function.21,24
A recent meta-analysis in rat models of middle cerebral artery occlusion (MCAO), showed that Sovateltide was linked with significantly lower neurological deficits and enhanced motor performance compared to control groups. The study reported no mortality in the Sovateltide group within 24 hours post-MCAO, while the control group exhibited a five-fold higher mortality risk. Additionally, Sovateltide administration led to reductions in infarct volume, improved differentiation potential of neuronal progenitor cells, and better mitochondrial outcomes. These findings highlight the promising neuroprotective and neurogenic effects of Sovateltide in animal models.25
Sovateltide represents a promising therapeutic agent for ischemic stroke due to its unique mechanism of action involving ETBR activation. By promoting neuroprotection, neuroregeneration, mitochondrial function, and NPC differentiation, Sovateltide has the potential to become a first-in-class drug for treating ischemic stroke. Its development underscores the importance of targeting specific receptors in the endothelin system to address complex neurovascular disorders effectively. Further studies are required to fully elucidate the molecular mechanisms underlying Sovateltide's effects on ETBR signalling and mitochondrial function in the brain after stroke.
The development of effective drugs for stroke is urgently required, as stroke is the second leading cause of death worldwide. Sovateltide, an endothelin B receptor (ETBR) agonist, has been studied as a potential therapeutic agent for treating acute cerebral ischemic stroke.
- Clinical Trials
- Phase I Trial
The Phase I clinical trial was aimed to establish the safety, tolerability, and pharmacodynamics of Sovateltide in healthy male volunteers. This open-label study involved three cohorts, each receiving multiple ascending doses of Sovateltide:
- Cohort 1 received three doses of 0.3 µg/kg at 4-hour intervals, totalling 0.9 µg/kg per day.
- Cohort 2 received three doses of 0.6 µg/kg at 4-hour intervals, totalling 1.8 µg/kg per day.
- Cohort 3 was planned to receive three doses of 0.9 µg/kg at 4-hour intervals, but dosing was stopped after the first participant experienced mild, transient adverse events (uneasiness, sweating, abdominal discomfort, vomiting) which resolved without intervention.
The study established 0.6 µg/kg as the Maximum Tolerated Dose (MTD) and 0.9 µg/kg as the Minimum Intolerable Dose (MID). No serious adverse effects were observed, indicating that Sovateltide is well tolerated and safe at the tested doses. The proposed therapeutic dose for subsequent studies was set at 0.3 µg/kg, below the MTD.8,16
- Phase II Trial
Building on the positive results from the Phase I trial, Phase II trials were initiated to evaluate the efficacy and safety of IRL-1620 in patients with cerebral ischemia and mild to moderate Alzheimer's disease.
The Phase II trial investigated the safety and efficacy of Sovateltide, as an adjunct therapy for acute cerebral ischemic stroke. This multicentre, randomized, double-blind, placebo-controlled study enrolled 40 patients who experienced ischemic stroke within 24 hours. Participants were divided into two groups, receiving either Sovateltide or a placebo (saline) alongside standard care. Sovateltide was administered at a dose of 0.3 µg/kg as an intravenous bolus over 1 min at an interval of 3 ± 1 h (total dose/day: 0.9 µg/kg) on days 1, 3, and 6. The study aimed to assess safety through monitoring adverse events and various health parameters while evaluating efficacy using the National Institute of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), and Barthel Index (BI) to measure neurological outcomes. Quality of life was also assessed using the EuroQoL-5 Dimensions (EQ-5D) and Stroke-Specific Quality of Life (SSQoL) scales.
The results showed that Sovateltide was well tolerated, with no significant drug-related adverse events reported. Patients treated with Sovateltide demonstrated significant improvements in neurological outcomes compared to the placebo group. Specifically, 64% of Sovateltide patients achieved a ≥ 40 points improvement on the BI by day 90, compared to 36% in the placebo group. Additionally, while the number of patients with a ≥ 2 points improvement on the mRS and a ≥ 6 points improvement on the NIHSS was numerically greater in the Sovateltide group (60% and 56%, respectively) compared to the placebo group (40% and 43%, respectively), these differences did not reach statistical significance at the 95% confidence level. Quality of life assessments using EQ-5D and SSQoL also indicated better outcomes for patients receiving Sovateltide. These findings suggest that Sovateltide has the potential to enhance recovery in stroke patients when used alongside standard care.26
- Phase III trial
In 2023, a randomized phase III clinical trial (NCT04047563) demonstrated that Sovateltide treatment significantly improved neurological outcomes for patients with acute cerebral ischemic stroke compared to a control group receiving normal saline. The study included patients aged 18 to 78 who presented within 24 hours of symptom onset and had radiologic confirmation of ischemic stroke. Exclusion criteria included intracranial haemorrhage and those undergoing endovascular therapy. Participants received standard care alongside either Sovateltide at a dose of 0.3 μg/kg or a saline placebo, administered three times as an IV bolus over 1 min at an interval of 3 h (± 1 h) on days 1,3, and 6. The primary objectives focused on assessing neurological outcomes using the mRS, NIHSS, and BI from day 1 to day 90.
The results showed that at 90 days, the median mRS was significantly lower in the Sovateltide group (1.00) compared to the control group (2.00), indicating better functional outcomes (p = 0.0078). An ordinal shift in mRS scores favoured Sovateltide, with 76% of patients achieving a score of 0–2 versus 54% in the control group. A significantly higher percentage of patients in the Sovateltide arm showed an improvement of ≥ 2 points on the mRS at 90 days compared to the control arm (76.12% vs. 52.86%; p = 0.0045). Additionally, the median NIHSS score was significantly lower in the Sovateltide group (1) than in the control group (3) at day 90, with more patients showing an improvement of ≥ 6 points on the NIHSS (82.09% vs. 64.29%; p = 0.019). The median BI score at day 90 was higher in the Sovateltide group (95.0) compared to the control group (85.0), indicating enhanced performance in daily activities (p = 0.0110), with an improvement of ≥ 40 points in BI observed in 76.12% of the Sovateltide group versus 61.43% in the control group.
Moreover, Sovateltide treatment significantly enhanced health-related quality of life as measured by EQ-5D-5L, with scores at day 90 being higher in the Sovateltide group (median score of 90 vs. 85 in the control group; p = 0.0055). Full recovery rates were also higher in the Sovateltide group, with 26.87%, 37.31%, and 38.81% of patients achieving full recovery according to mRS, NIHSS, and BI scores, respectively, compared to 15.71%, 25.71%, and 28.57% in the control group. Baseline characteristics, including the extent of the infarction and timing of treatment initiation, were similar between both groups, supporting the efficacy of Sovateltide in improving outcomes for acute ischemic stroke patients.8,27
- Pharmacokinetics
Sovateltide is administered intravenously, allowing for rapid entry into the bloodstream and distribution throughout the body. This route is critical for acute conditions like ischemic stroke to ensure quick therapeutic action. In a clinical trial involving cancer patients, Sovateltide exhibited dose-proportional increases in maximum plasma concentration (Cmax) and greater-than-dose-proportional increases in the area under the concentration-time curve (AUC) when administered at doses ranging from 0.05 to 15.1 μg/m² over one minute. Sovateltide is 83-85% bound to plasma proteins, suggesting a potential for interaction with other drugs that also bind to plasma proteins. This high binding affinity influences its distribution and bioavailability. The volume of distribution in cancer patients was approximately equal to the intravascular volume, indicating limited distribution beyond the blood plasma.8
The elimination half-life of Sovateltide ranged from 4.38 to 8.29 minutes, necessitating multiple dosing to maintain therapeutic levels. Sovateltide exhibited low systemic clearance, suggesting efficient utilization and metabolism. In vitro studies show that Sovateltide has low hepatic intrinsic clearance and does not inhibit or induce major cytochrome P450 enzymes such as CYP1A2, CYP2B6, or CYP3A4. This profile suggests minimal interference with common metabolic pathways.8,9
Sovateltide inhibits organic anion-transporting polypeptides (OATP1B1 and OATP1B3), which may affect its hepatic uptake, but it does not significantly inhibit other transporters. It exhibits low permeability across cell membranes.8,9
Dose and route of administration
In the above clinical trials described, Sovateltide was administered at a dose of 0.3 μg/kg. The route of administration was intravenous (IV), given as a bolus injection over one minute. The treatment regimen consisted of administering the dose three times, with each dose given at an interval of 3 hours (± 1 hour) on days 1, 3, and 6. This resulted in a total daily dose of 0.9 μg/kg on each of those treatment days.8,16,26
- Safety profile
In the Phase I trial, the first participant who received a dose of 0.9 µg/kg experienced mild and transient adverse events, such as uneasiness, sweating, abdominal discomfort, and vomiting, which resolved without intervention. No serious adverse effects were observed, indicating that Sovateltide is well-tolerated and safe at the tested doses. No adverse events occurred in the 0.3 µg/kg and 0.6 µg/kg groups.26
In the randomized phase III trial involving patients with acute cerebral ischemic stroke, Sovateltide was generally well tolerated, with no drug-related adverse events reported. Mild treatment-emergent adverse events (TEAEs) occurred in 3 of 80 Sovateltide recipients and 6 of 78 control patients, with abdominal pain being the most common in the control group. Moderate TEAEs were reported in 7 Sovateltide recipients and 16 control patients, with common symptoms including fever, hypertension, cold, vomiting, and dizziness. Serious TEAEs were reported in 5 patients in the Sovateltide group (including 4 deaths and 1 case of hyponatremia) and in 2 patients in the control group (both resulting in death).8
Overall, Sovateltide showed a favourable safety profile with no drug-related adverse effects, and the incidence of TEAEs was comparable between the treatment and control groups.
- Approval for use
Sovateltide, received its first regulatory approval in India on May 31, 2023 (sold in India under the brand name Tyvalzi), for the treatment of patients with ischemic stroke within 24 hours of symptom onset. Exclusion criteria for the treatment include patients with intracranial haemorrhage and those undergoing endovascular therapy. These criteria ensure that Sovateltide is used for patients who can benefit most from its neuroprotective effects. This approval marks a significant advancement in stroke care, providing a new therapeutic option for patients in India.28
- Ongoing trials
NCT05691244 is a multicentre, randomized, double-blind, placebo-controlled phase III study sponsored by Pharmazz, Inc. This trial aims to evaluate the safety and efficacy of Sovateltide in treating patients with acute cerebral ischemic stroke. The study plans to enrol 514 participants across the United States, Canada, the United Kingdom, and Europe, where similar demographics and standards of care are expected to provide consistent data. Eligible participants are aged 18 to 80, must present within 24 hours of stroke symptom onset, and have a radiologically confirmed ischemic stroke with a National Institutes of Health Stroke Scale (NIHSS) score between 8 and 20 and a Level of Consciousness (1A) score of less than 2. The trial excludes individuals with intracranial haemorrhage or those receiving endovascular therapy. Participants will be randomly assigned to receive either Sovateltide or a placebo intravenously. The study start date is November 2024, with an estimated primary completion date in October 2026 and overall study completion by November 2026. This research is crucial in determining whether Sovateltide can significantly enhance recovery and improve functional outcomes in stroke patients.29
Another clinical trial (NCT05955326) is a phase III, multicentre, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of Sovateltide in adult patients with cerebral ischemic stroke in India. Sponsored by Pharmazz, Inc., the trial plans to enrol 160 participants aged 18 to 75 who have been clinically diagnosed with acute ischemic stroke and present within 24 hours of symptom onset, with an NIHSS score between 8 and 18. Patients with intracranial haemorrhage, recent stroke history, or those participating in another clinical trial within the past 30 days are excluded. The study involves administering Sovateltide or a placebo intravenously, with the primary outcome being the proportion of patients achieving a favourable outcome, defined as a mRS score of 0-2, on day 90 post-randomization. The trial commenced in January 2024 and is expected to complete by 2025. This study aims to provide robust data on Sovateltide’s potential to enhance recovery and improve neurological outcomes in patients experiencing acute cerebral ischemic stroke.30
- Sovateltide in other diseases
Targeting amyloid in Alzheimer's patients may not sufficiently halt disease progression. Research is increasingly focusing on repair mechanisms and biochemical cascades that promote cell survival and regeneration. This approach involves enhancing neurogenesis and angiogenesis, which are critical for forming new neurons and ensuring adequate blood supply. Sovateltide, has shown promise in preclinical studies by improving neuronal progenitor cell differentiation, mitochondrial function, and enhancing neurogenesis and angiogenesis, leading to improved learning and memory in Alzheimer's animal models.9
Hypoxic-ischemic encephalopathy (HIE) is a severe complication from oxygen deprivation at birth, leading to significant neonatal mortality and lifelong disabilities. Current treatments like therapeutic hypothermia have limitations, necessitating new therapies. Sovateltide has demonstrated significant neuroprotective and neuroregenerative effects in HIE animal models, reducing apoptosis, enhancing cerebral blood flow, and promoting neurogenesis and angiogenesis. These promising results suggest potential therapeutic benefits in HIE, leading to planned Phase I studies in neonates. Additionally, Sovateltide's ability to augment neurogenesis and improve motor function recovery after spinal cord injury is under investigation, with a Phase II study ongoing for patients with acute spinal cord injuries. This research indicates Sovateltide's potential across various neurological conditions, offering hope for improved outcomes in complex diseases (Figure 2).9
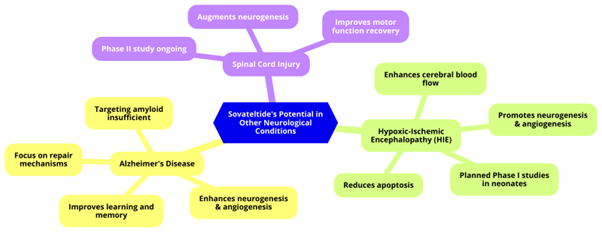
Figure 2: Sovateltide's Potential in Other Neurological Conditions
Future Directions in Sovateltide Research and Application
Given the promising results of Sovateltide in preclinical and clinical studies, future research should focus on further elucidating its mechanisms of action and optimizing its therapeutic use across a range of neurological conditions. One important direction is to conduct large-scale, multicentre clinical trials that extend beyond the current time constraints of acute stroke treatments. Such studies should aim to establish the efficacy of Sovateltide in improving outcomes for patients who are unable to receive traditional reperfusion therapies due to delayed hospital arrival or contraindications. Additionally, research should explore the potential of Sovateltide to be used as an adjunctive therapy alongside existing treatments like thrombolysis and thrombectomy, potentially widening the therapeutic window and enhancing recovery. This includes investigating optimal dosing regimens, administration timing, and combination with other neuroprotective agents to maximize the drug’s benefits.
Another promising area for future research involves exploring the application of Sovateltide in other neurodegenerative and neurovascular disorders. With its demonstrated effects on neuroprotection, neuroregeneration, and angiogenesis, Sovateltide could have significant therapeutic implications for conditions such as Alzheimer’s disease, hypoxic-ischemic encephalopathy (HIE), and spinal cord injuries. Preclinical studies have already shown Sovateltide's ability to improve cognitive function and promote neurogenesis in Alzheimer’s models, as well as enhance recovery after spinal cord injuries. Future studies should include well-designed clinical trials to evaluate Sovateltide's safety and efficacy in these contexts, with an emphasis on understanding its role in cell survival pathways and mitochondrial function. Moreover, exploring biomarkers of response and developing patient stratification strategies could further refine the therapeutic use of Sovateltide, ultimately leading to personalized treatment approaches in stroke care and beyond.
Conclusion
Stroke remains a leading cause of death and long-term disability globally, with ischemic stroke accounting for most cases. Current treatments, such as tPA and mechanical thrombectomy, are effective but are limited by narrow therapeutic windows and strict eligibility criteria. Consequently, many patients do not receive timely intervention, highlighting the need for alternative therapeutic strategies that extend beyond acute treatment windows. Sovateltide, a novel endothelin B receptor agonist, offers a promising new approach to ischemic stroke therapy. By targeting neuroprotection, neurogenesis, and angiogenesis, Sovateltide provides a comprehensive mechanism of action that addresses the complex pathophysiology of stroke, offering potential benefits for a broader range of patients, including those who cannot access traditional reperfusion therapies.
Clinical trials have demonstrated Sovateltide's efficacy and safety, with significant improvements in neurological outcomes and quality of life measures. These findings suggest that Sovateltide could significantly extend the treatment window and improve recovery in stroke patients. Future research should focus on large-scale clinical trials to validate these results and explore Sovateltide's application in other neurodegenerative and neurovascular disorders. Optimizing dosing regimens, exploring combination therapies, and identifying biomarkers for patient stratification are crucial for maximizing Sovateltide's therapeutic potential. With continued research and clinical evaluation, Sovateltide has the potential to transform stroke treatment, offering hope for improved patient outcomes and new therapeutic paradigms in ischemic stroke care.
References
- Johnson, C. O., Nguyen, M., Roth, G. A., Nichols, E., Alam, T., Abate, D., Abbafati, C., Abd-Allah, F., Abdelalim, A., Abraha, H. N., Abu-Raddad, L. J., Abu-Rmeileh, N. M. E., Abualhasan, A., Alvis-Guzman, N., Amare, A. T., Amini, E., Ammar, W., Anber, N. H., Anwari, P., … Murray, C. J. L. (2019). Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology, 18(5), 439–458. https://doi.org/10.1016/S1474-4422(19)30034-1
- Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res. 2017;120(3):439-448. doi:10.1161/CIRCRESAHA.116.308413
- Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129-142. doi:10.1016/S0140-6736(20)31179-X
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association [published correction appears in Stroke. 2019 Dec;50(12):e440-e441. doi: 10.1161/STR.0000000000000215]. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708-718. doi:10.1056/NEJMoa1713973
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11-21. doi:10.1056/NEJMoa1706442
- Kobeissi H, Ghozy S, Adusumilli G, et al. Endovascular Therapy for Stroke Presenting Beyond 24 Hours: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6(5):e2311768. Published 2023 May 1. doi:10.1001/jamanetworkopen.2023.11768
- Keam SJ. Sovateltide: First Approval. Drugs. 2023;83(13):1239-1244. doi:10.1007/s40265-023-01922-4
- Ranjan AK, Gulati A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. Int J Mol Sci. 2022;23(6):3146. Published 2022 Mar 15. doi:10.3390/ijms23063146
- Lampl Y, Fleminger G, Gilad R, Galron R, Sarova-Pinhas I, Sokolovsky M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke. 1997;28(10):1951-1955. doi:10.1161/01.str.28.10.1951
- Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23(7):1014-1016. doi:10.1161/01.str.23.7.1014
- Chuquet J, Benchenane K, Toutain J, MacKenzie ET, Roussel S, Touzani O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke. 2002;33(12):3019-3025. doi:10.1161/01.str.0000039401.48915.9f
- Ehrenreich H, Oldenburg J, Hasselblatt M, et al. Endothelin B receptor-deficient rats as a subtraction model to study the cerebral endothelin system. Neuroscience. 1999;91(3):1067-1075. doi:10.1016/s0306-4522(98)00663-0
- Ranjan AK, Briyal S, Gulati A. Sovateltide (IRL-1620) activates neuronal differentiation and prevents mitochondrial dysfunction in adult mammalian brains following stroke. Sci Rep. 2020;10(1):12737. Published 2020 Jul 29. doi:10.1038/s41598-020-69673-w
- Cifuentes EG, Hornick MG, Havalad S, Donovan RL, Gulati A. Neuroprotective Effect of IRL-1620, an Endothelin B Receptor Agonist, on a Pediatric Rat Model of Middle Cerebral Artery Occlusion. Front Pediatr. 2018;6:310. Published 2018 Oct 23. doi:10.3389/fped.2018.00310
- Gulati A, Hornick MG, Briyal S, Lavhale MS. A novel neuroregenerative approach using ET(B) receptor agonist, IRL-1620, to treat CNS disorders. Physiol Res. 2018;67(Suppl 1):S95-S113. doi:10.33549/physiolres.933859
- Leonard MG, Briyal S, Gulati A. Endothelin B receptor agonist, IRL-1620, reduces neurological damage following permanent middle cerebral artery occlusion in rats. Brain Res. 2011;1420:48-58. doi:10.1016/j.brainres.2011.08.075
- Leonard MG, Briyal S, Gulati A. Endothelin B receptor agonist, IRL-1620, provides long-term neuroprotection in cerebral ischemia in rats. Brain Res. 2012;1464:14-23. doi:10.1016/j.brainres.2012.05.005
- Joshi MD, Oesterling BM, Wu C, et al. Evaluation of liposomal nanocarriers loaded with ETB receptor agonist, IRL-1620, using cell-based assays. Neuroscience. 2016;312:141-152. doi:10.1016/j.neuroscience.2015.11.016
- Ranjan AK, Briyal S, Khandekar D, Gulati A. Sovateltide (IRL-1620) affects neuronal progenitors and prevents cerebral tissue damage after ischemic stroke. Can J Physiol Pharmacol. 2020;98(9):659-666. doi:10.1139/cjpp-2020-0164
- Gulati A. Endothelin Receptors, Mitochondria and Neurogenesis in Cerebral Ischemia. Curr Neuropharmacol. 2016;14(6):619-626. doi:10.2174/1570159x14666160119094959
- Koyama Y, Nagae R, Tokuyama S, Tanaka K. I.c.v administration of an endothelin ET(B) receptor agonist stimulates vascular endothelial growth factor-A production and activates vascular endothelial growth factor receptors in rat brain. Neuroscience. 2011;192:689-698. doi:10.1016/j.neuroscience.2011.05.058
- Leonard MG, Gulati A. Endothelin B receptor agonist, IRL-1620, enhances angiogenesis and neurogenesis following cerebral ischemia in rats. Brain Res. 2013;1528:28-41. doi:10.1016/j.brainres.2013.07.002
- Briyal S, Ranjan AK, Hornick MG, Puppala AK, Luu T, Gulati A. Anti-apoptotic activity of ETB receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia [published correction appears in Sci Rep. 2020 Feb 14;10(1):2992. doi: 10.1038/s41598-020-60114-2]. Sci Rep. 2019;9(1):10439. Published 2019 Jul 18. doi:10.1038/s41598-019-46203-x
- Moustakas D, Mani I, Pouliakis A, Iacovidou N, Xanthos T. The Effects of IRL-1620 in Post-ischemic Brain Injury: A Systematic Review and Meta-analysis of Experimental Studies. Neurocrit Care. Published online May 9, 2024. doi:10.1007/s12028-024-01994-4
- Gulati A, Agrawal N, Vibha D, et al. Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. CNS Drugs. 2021;35(1):85-104. doi:10.1007/s40263-020-00783-9
- Cerebral Ischemic Stroke | Lyfaquin® | Pharmazz, Inc. Accessed August 05, 2024. https://www.pharmazz.com/cerebral-ischemic-stroke.php
- Press Releases | Pharmazz, Inc. Accessed August 05, 2024. https://www.pharmazz.com/press-releases.php
- ClinicalTrials.gov. Accessed August 07, 2024. https://clinicaltrials.gov/study/NCT05691244
- ClinicalTrials.gov. Accessed August 07, 2024. https://clinicaltrials.gov/study/NCT05955326